Analyzing Exhaust Emissions

An engine's combustion is analyzed by measuring the levels of five gases in the exhaust stream. The five gases we monitor are HC, NOx , CO, CO2 , and O2 . Carbon dioxide or CO2 is the product of perfect combustion. Unfortunately, even CO2 is considered a greenhouse gas. The greenhouse gases are carbon dioxide, nitrous oxide, methane, and chlorofluorocarbons.
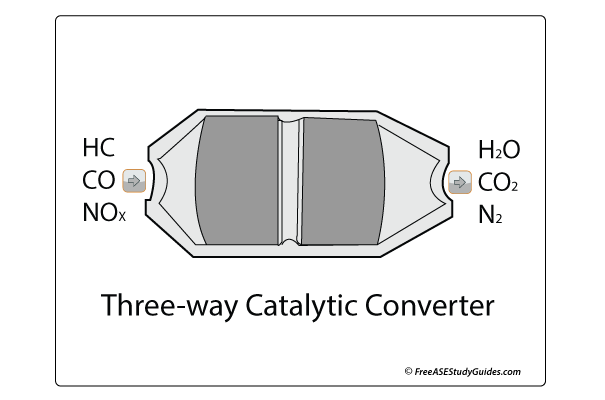
By monitoring these five gases, we know what's happening inside and sometimes outside the combustion chamber. HC's, NOx , and CO are considered pollutants.
HC's or hydrocarbons are leftover, unburned fuel found in the cylinder after incomplete combustion. All engines have a little excess fuel left over after ignition; the question is how much is acceptable. Rich air-fuel mixtures result in elevated HCs because the mixture has too much fuel. Too lean of an air-fuel ratio will also cause excessive HCs in exhaust emissions. This problem is called lean burn and results from the misfire caused by insufficient fuel in the mixture. Unburned fuel entering the catalytic converter overheats and damages the substrate.

NOx High temperatures in the combustion chamber cause NOx or oxides of nitrogen. Oxygen and nitrogen combine in the combustion chamber at 2500° F or higher temperatures to create this pollutant. Both oxygen and nitrogen enter the combustion chamber; the key is to keep cylinder temperatures below this crucial 2500° F to prevent producing this harmful gas. NOx is a pollutant that causes smog. An overheating engine, faulty EGR valve, and a lean air-fuel ratio aid in creating NOx.

CO or carbon monoxide is considered a harmful gas and a byproduct of combustion. It occurs when carbon and oxygen combine because the mixture has too much fuel, and the air-fuel ratio is rich. When an engine is running correctly, CO levels are low. A leaking fuel injector, high fuel pressure, or restricted airflow, like a clogged air filter or plugged PCV system, can result in excessive carbon monoxide.