Battery Specific Gravity Test

The lead-acid battery used in today's automobile is made of plates, lead, and lead oxide in an electrolyte solution. This solution consists of 65% water and 35% sulfuric acid. This solution's specific gravity or weight increases as the battery charges and decreases as the battery discharges. As the battery discharges, sulfur moves away from the solution and toward the plates. The opposite is true as the battery is charged. The sulfur returns to the electrolyte solution.
The specific gravity of the electrolyte depends on this 65% to 35% ratio for the necessary chemical reaction to occur. This ratio is affected by the amount of sulfuric acid and the temperature of the solution.
As the temperature drops, the electrolyte contracts, increasing the specific gravity. As the temperature increases, the electrolyte expands, deviating from its optimal ratio and affecting the specific gravity reading.
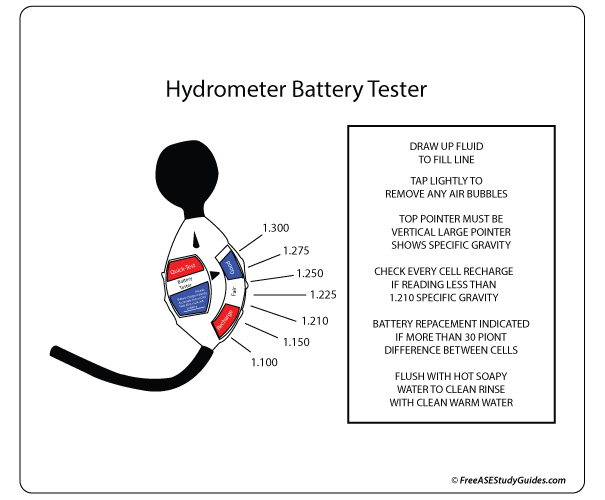
A battery's specific gravity is a great way of measuring a battery's state of charge. This is because, during discharge, the specific gravity decreases linearly with ampere-hours discharged. The specific gravity also increases as the battery is recharged.
A hydrometer measures the specific gravity of the electrolyte solution in each cell. It's a tool used to measure the density or weight of a liquid compared to the density of an equal amount of water. A lead-acid battery cell is fully charged with a specific gravity of 1.265 at 80° F. For temperature adjustments, get a specific gravity reading and adjust to temperature by adding .004 for every 10° F above 80° F and subtracting .004 for every 10° F below 80° F.